Which of these statements best describes a dative covalent bond. Which statement below best describes a polar covalent bond.

Which Statement Best Describes Covalent Bonding Brainly Com
5 Which statement best.

. The bonds that are formed are strong and require a lot of energy to break them. Which statement provides the best evidence to support the claim that Burmese pythons have an impact on the environment. Protons have a charge of.
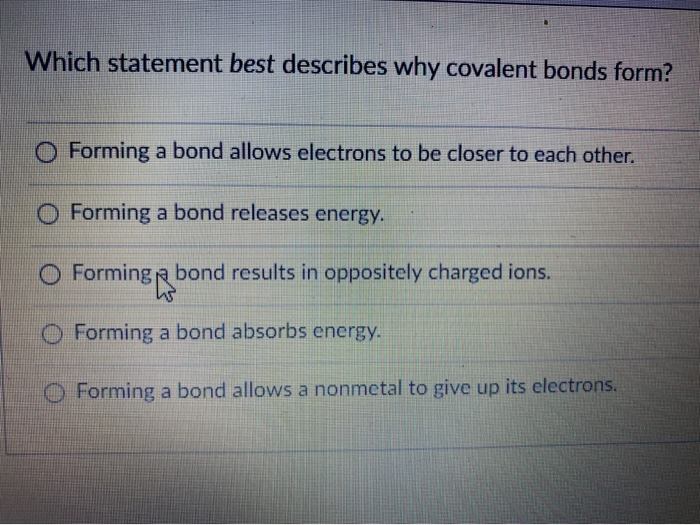
Forming bond results in oppositely charged ions. 3 Which characteristic best explains why carbon is important to living organisms. - equal sharing of electrons.
Answer choices -1 1-2 2. A In ionic bonds electrons are shared while in covalent bonds electrons are ripped between atoms. C In ionic bonds electrons are ripped while in covalent bonds electrons are shared between atoms D.
O Forming a bond releases energy. What are the two type of covalent bonding. The spear action of electric charge to a molecule to show a molecular dipole.
Which statement correctly describes a covalent bond. Valence electrons are transferred from one atom to the other. Which of the following describes covalent bonds.
When two non-metal atoms share a pair of electrons a covalent bond is formed. Polar covalent bonds form when two atoms share electrons unequally with one electron spending more time around one atom than. Statement 4 covalent bonding involves the sharing of electrons.
One pure covalent bond is formed when two atoms with the same electronegativitie s come together. - electronegativity is 0-04. All of the atoms electrons are shared between the atoms please help ℹ lydia Aug 30 2017.
The atoms valence electrons are shared between the atoms. Which statement best describes why covalent bonds form. The bonding is best described as covalent and because of the difference in electronegativity the bond is polar.
Like a result a pure covalent bond lacks no ionic property. A pair of electrons shared between two atoms where one atom has donated two electrons. The outer shell of an atom would be completed.
- even distribution of chargeno dipole. Covalent bonds are magical. Polar covalent bonds form when two atoms share electrons equally with electrons spend the same amount of time around each atom and no partial charges are formed.
1 Which Statement Best Explains Why Carbon Is Present. Bonds form because of opposite charges. 4 Which reason best explains why carbon is able to form macromolecules quizlet.
The electrons involved are located in the atoms outer shells. Forming a bond allows electrons to be closer to each other. B In ionic bonds and covalent bonds electrons are shared.
Statement 3 covalent bonding involves the sharing of electrons. A pair of electrons shared between two atoms where each atom has donated one electron. Forming a bond releases energy.
What is covalent bonding. 2 Which statement best explains why carbon is present in so many kinds of molecules it can form four covalent bonds. Forming a bond allows electrons to be closer to each other.
I think its a or b Which scenario describes periodic behavior. A statement that can be expressed in if-then form is a 1 point A. A Statement 1 B statement 2 C statement 3 or.
Two pairs of electrons shared between two atoms where each atom has donated one electron. The atoms valence electrons combine to form a network of bonds. Which statement best describes why covalent bonds form.
Forming a bond absorbs energy. The bonds that are formed are weak and do not require much energy to break them. Forming a bond absorbs energy.
Formingp bond results in oppositely charged ions. Electrons are transferred between atoms. Forming a bond allows a.
Bonds form to fill outer electron shells.

Covalent Bond Definition Types And Examples
Solved Which Statement Best Describes Why Covalent Bonds Chegg Com

Covalent Bond Definition Types And Examples

Covalent Bond Definition Properties Examples Facts Britannica
0 Comments